|
Publications |

|
PASINI RESEARCH GROUP |
|
ORGANIC, SUPRAMOLECULAR AND POLYMERIC MATERIALS |

|
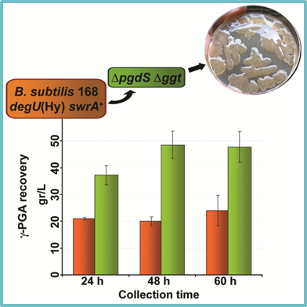
76. Knockout of pgdS and ggt genes improves γ-PGA yield in B. subtilis, V. Scoffone, D. Dondi, G. Biino, G. Borghese, D. Pasini, A. Galizzi, C. Calvio,* Biotechnol. Bioeng., 2013, 110, 2006-2012. doi: 10.1002/bit.24846 |
|
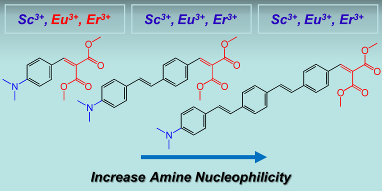
77. From Red to Blue Shift: Switching the Binding Affinity from the Acceptor to the Donor End by Increasing the p-Bridge in Push-Pull Chromophores with Coordinative Ends, M. Caricato, C. Coluccini, D. A. Vander Griend, A. Forni, D. Pasini,* New J. Chem., 2013, 37, 2792-2799. doi: 10.1039/C3NJ00466J |
|
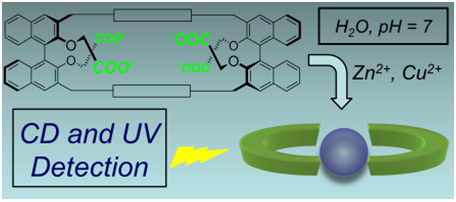
78. A Chiroptical Probe for Sensing Metal Ions in Water, M. Caricato, N. J. Leza, K. Roy, D. Dondi, G. Gattuso, L. S. Shimizu, D. A. Vander Griend, D. Pasini,* Eur. J. Org. Chem., 2013, 6078-6083. doi: 10.1002/ejoc.201300884 |
|
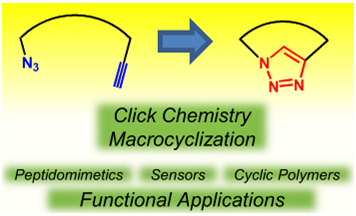
79. The Click Reaction as an Efficient Tool for the Construction of Macrocyclic Structures, D. Pasini,* Molecules, 2013, 9512-9530. (Invited contribution to the Special Issue: “Advances in Click Chemistry” – Open Access Publication Fees Waived) doi: 10.3390/molecules18089512 |
|
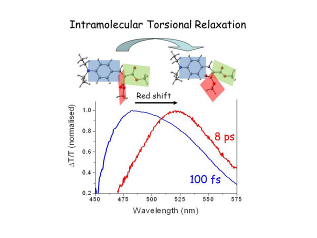
80. Direct evidence of torsional motion in an aggregation-induced emissive chromophore, T. Virgili,* A. Forni,* E. Cariati, D. Pasini, C. Botta, J. Phys. Chem. C, 2013, 51, 27161-27166. doi: 10.1021/jp4104504 |
|
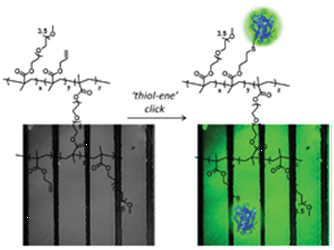
81. ‘Clickable’ hydrogels for all: facile fabrication and functionalization, L. Beria, T. N. Gevrek, A. Erdog, R. Sanyal, D. Pasini,* A. Sanyal,* Biomat. Sci., 2014, 2, 67-75 . doi: 10.1039/C3BM60171D |
|
83. ‘Nanostructuring with Chirality: Binaphthyl-Based Synthons for the Production of Functional Oriented Nanomaterials, M. Caricato, A. K. Sharma, C. Coluccini, D. Pasini,* Nanoscale, 2014, 6, 7165-7174. doi: 10.1039/C4NR00801D |
|
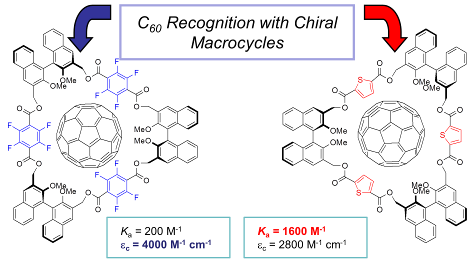
82. ‘Homochiral BINOL-based macrocycles with p-electron-rich, electron-withdrawing or extended spacing units as receptors for C60, M. Caricato, S Díez González, I. Arandia Ariño, D. Pasini,* Beilstein J. Org. Chem., 2014, 10, 1308-1316. (Invited contribution to the Thematic Series: Functionalized carbon-nanomaterials, Editor Prof. Anke Krueger) doi: 10.3762/bjoc.10.132 |
|
85. ‘Crystal structure analyses facilitate understanding of synthesis protocols in the preparation of 6,6′-dibromo-substituted BINOL compounds’, M. Agnes, A. Sorrenti, D. Pasini,* K. Wurst, D. B. Amabilino,* CrystEngComm, 2014, 16, 10131-10138 . doi: 10.1039/C4CE01160K |
|
84. ‘Stereospecific generation of homochiral helices in coordination polymers built from enantiopure binaphthyl-based ligands’, M. Crespo Alonso, M. Arca, F. Isaia, R. Lai, V. Lippolis, S. K. Callear, M. Caricato, D. Pasini,* S. J. Coles, M. C. Aragoni,* CrystEngComm, 2014, 16, 8582-8590. doi: 10.1039/C4CE01101E |
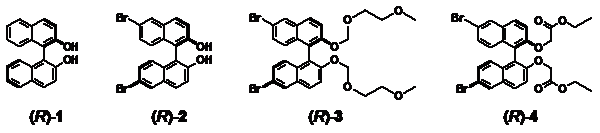
|
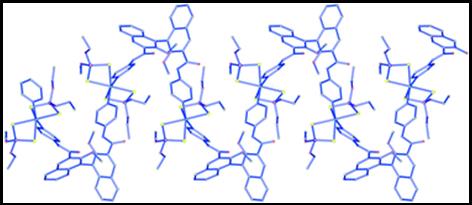
86. ‘Chiral Nanostructuring of Multivalent Macrocycles in Solution and on Surfaces’, M. Caricato, A. Deforge, D. Bonifazi, D. Dondi, A. Mazzanti, D. Pasini,* Org. Biomol. Chem., 2015, 13, 3593-3601. (Highlighted as “Hot Paper 2015”) doi: 10.1039/C4OB02643H |
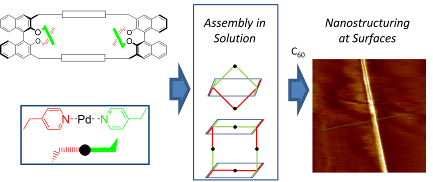
|
87. ‘Synthesis, chiroptical and SHG properties of polarizable push-pull dyes built on π-extended binaphthyls’, C. Coluccini, M. Caricato, E. Cariati, S. Righetto, A. Forni, D. Pasini,* RSC Advances, 2015, 5, 21495-21503. doi: 10.1039/c4ra16876c |
|
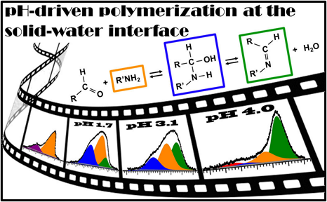
89. ‘Surface-Enhanced Polymerization via Schiff-Base Coupling at the Solid-Water Interface under pH Control’, M. Di Giovannantonio, T. Kosmala, B. Bonanni, G. Serrano, N. Zema, S. Turchini, D. Catone, K. Wandelt, D. Pasini, G. Contini,* C. Goletti, J. Phys. Chem. C, 2015, 119, 19228-19235. Included in the Elettra Highlights 2015-2016 booklet. doi: 10.1021/acs.jpcc.5b05547 |
|
90. ‘Synthesis of Binaphthyl-Based Push-Pull Chromophores with Supramolecularly Polarizable Acceptor Ends’, C. Coluccini,* G. Terraneo, D. Pasini,* J. Chem., 2015 Article Number: 827592. Invited contribution from the Journal’s Editorial Board. doi: 10.1155/2015/827592 |
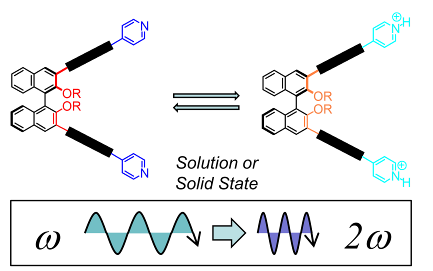
|
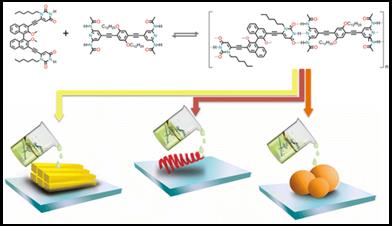
88. ‘Solvent Molding of Organic Morphologies Made of Supramolecular Chiral Polymer’, L. Dordević, T. Marangoni, T. Miletić, J. Rubio-Magnieto, J. Mohanraj, H. Amenitsch, D. Pasini, N. Liaros, S. Couris, N. Armaroli,* M. Surin,* D. Bonifazi,* J. Am. Chem. Soc. 2015, 137, 8150-8160. doi: 10.1021/jacs.5b02448 |
|
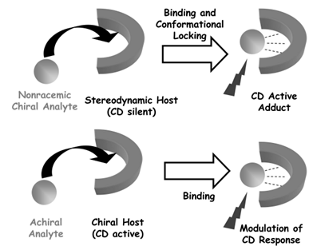
91. ‘Recent Advances in Chirality Sensing using Atropoisomeric Molecular Receptors, D. Pasini,* A. Nitti, Chirality, 2016, 28, 116-123. Invited contribution from the Journal’s Editorial Board. Special Issue in honour of Francesco Gasparrini. doi: 10.1002/chir.22556 |
|
92. ‘Microstructured chitosan/poly(γ-glutamic acid) polyelectrolyte complex hydrogels by computer-aided wet-spinning for biomedical three-dimensional scaffolds’, D. Puppi, C. Migone, A. Morelli, C. Bartoli, M. Gazzarri, D. Pasini, F. Chiellini,* J. Bioact. Compat. Polym., 2016, 31, 531–549. doi: 10.1177/0883911516631355 |
|
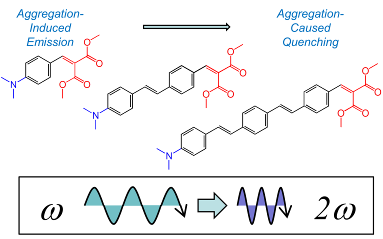
93. ‘Polymorphism-Dependent Aggregation Induced Emission of a Push-Pull Dye and its Multi-Stimuli Responsive Behavior’, C. Botta,* S. Benedini, L. Carlucci, A. Forni,* D. Marinotto, A. Nitti, D. Pasini,* S. Righetto, E. Cariati* J. Mat. Chem. C 2016, 4, 2979-2989. doi: 10.1039/C5TC03352G |
|
75. Switching of Emissive and NLO Properties in Push-Pull Chromophores with Crescent PPV-like Structures, C. Coluccini, A. K. Sharma, M. Caricato, A. Sironi, E. Cariati,* S. Righetto, E. Tordin, C. Botta,* A. Forni,* D. Pasini,* Phys. Chem. Chem. Phys., 2013, 15, 1666-1674. doi: 10.1039/C2CP43140H |
|
97. ‘Structure-Activity Relationships for the Solid State Emission of a New Family of “Push-Pull” p-Extended Chromophores’, A. Nitti, F. Villafiorita-Monteleone, A. Pacini, C. Botta, T. Virgili, A. Forni, E. Cariati, M. Boiocchi, D. Pasini,* Faraday Discuss. 2017, 196, 455-460. doi: 10.1039/C6FD00161K |
|
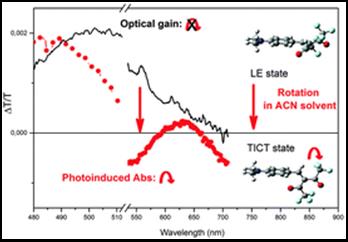
94. ‘Long-living optical gain induced by solvent viscosity in a push–pull molecule, M. Mroz, S. Benedini, A. Forni,* D. Pasini, E. Cariati, T. Virgili,* Phys. Chem. Chem. Phys. 2016, 18, 18289-18296. doi: 10.1039/C6CP02988D |
|
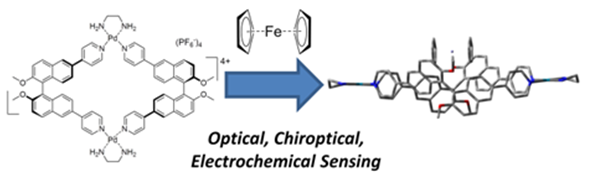
95. ‘A chiroptical molecular sensor for ferrocene, M. Agnes, A. Nitti, D. A. Vander Griend, D. Dondi, D. Merli, D. Pasini,* Chem. Commun., 2016, 52, 11492-11495. (Cover) doi: 10.1039/C6CC05937F |
|
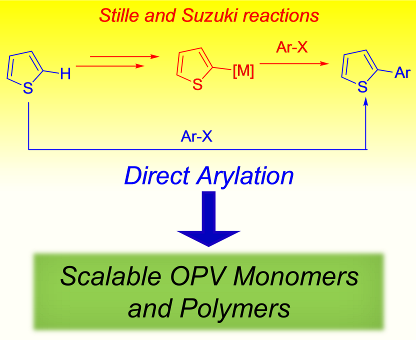
102. ‘Direct Arylation Strategies in the Synthesis of pi-Extended Monomers for Organic Polymeric Solar Cells, A. Nitti, G. Bianchi, R. Po, D. Pasini,* Molecules 2017, 22, 21. Invited contribution – Open Access Publication Fees Waived. doi: 10.3390/molecules22010021 |
|
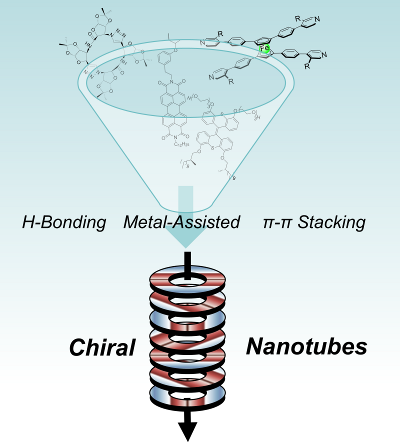
104. ‘Chiral nanotubes, A. Nitti, A. Pacini, D. Pasini,* Nanomaterials, 2017, 7, 167. Invited contribution – Open Access Publication Fees Waived. doi: 10.3390/nano7070167 |
|
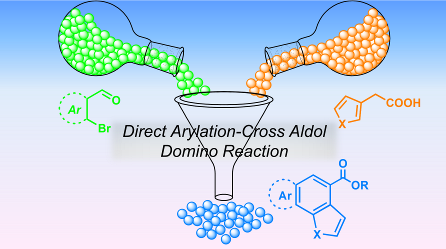
105. ‘Domino Direct Arylation and Cross-Aldol for Rapid Construction of Extended Polycyclic π-Scaffolds’, A. Nitti, G. Bianchi, R. Po, T. M. Swager, D. Pasini,* J. Am. Chem. Soc., 2017, 139, 8788-8791. doi: 10.1021/jacs.7b03412. |
|
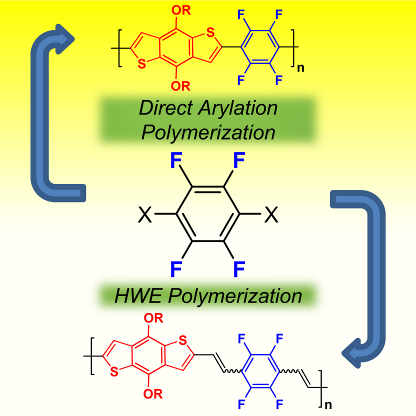
103. ‘Donor–acceptor conjugated copolymers incorporating tetrafluorobenzene as the π-electron deficient unit’, A. Nitti, F. Debattista, L. Abbondanza, G. Bianchi, R. Po, D. Pasini,* J. Polym. Sci. Pol. Chem., 2017, 55, 1601-1610. doi: 10.1002/pola.28532 |
|
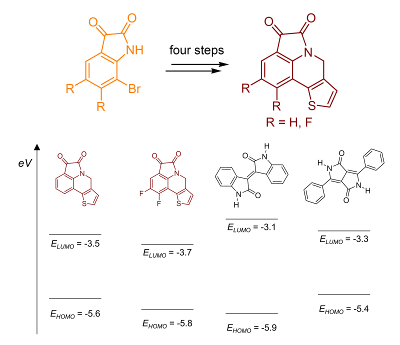
96. ‘Conjugated Thiophene-Fused Isatin Dyes through Intramolecular Direct Arylation, A. Nitti, F. Villafiorita-Monteleone, A. Nitti, M. Signorile, M. Boiocchi, G. Bianchi, R. Po, D. Pasini,* J. Org. Chem. 2016, 81, 11035-11042 . doi:10.1021/acs.joc.6b01922 |
|
98. ‘Optoelectronic devices of highly efficient luminogens in the solid state: general discussion’, S. Wu, X. He, B. Liu, R. Hu, Z. Li, G. Krishnamoorthy, A. Qin, Y. Tang, D. Pasini, Y. Tsuchiya, T. Jadhav, Z. Zhao, H. Peng, H. Tian, J. Z. Sun, M.-Q. Zhu, Y. Ma, B. Z. Tang, Faraday Discuss. 2017, 196, 455-460. doi: 10.1039/C7FD90004J |
|
99. ‘New and efficient fluorescent and phosphorescent luminogens: general discussion’, SN. Leung, A. Pucci, R. Hu, E. E. B. Campbell, G. Krishnamoorthy, B. Z. Tang, S. Wu, F. Zhang, J. Mei, W. Bai, B. Li, X. He, Y. Tang, B. Liu, R. Zhang, Z. Wang, A. Qin, Z. Li, D. Zhang, D. Pasini, W. Tian, Y. Tsuchiya, T. Jadhav, Y. Wang, Z. Zhao, G. He, K. Li, E. Rivard, M.-Q. Zhu, B. Xu, J. Z. Sun, Y. Chujo, J.-L. Hong, L. Kong, P. Lu, C.-C. Chang, K. Wang, R. A. Singh, Faraday Discuss. 2017, 196, 191-218. doi: 10.1039/C7FD90003A |
|
100. Advanced functional luminogens in the solid-state: general discussion, A. Pucci, N. Leung, A. Hor, S. Wu, Y. Gu, B. Li, B. Liu, R. Hu, A. Qin, X. Chen, Z. Li, D. Zhang, G. Krishnamoorthy, D. Pasini, Y. Tsuchiya, K. Wang, X. He, H. Peng, Z. Zhao, G. He, B. Z. Tang, E. Rivard, F. Ito, H. Tian, M.-Q. Zhu, J. Z. Sun, Y. Chujo, G. Kuang, Y. Ma, W. Tian, B. Xu, O. Tsutsumi, P. Duan, Faraday Discuss. 2017, 196, 317-334. doi:10.1039/C7FD90001E |
|
101. Biomedical applications of luminogens: general discussion, A. Pucci, A. Hor, M. Gao, S. Wu, Y. Yu, B. Li, X. He, B. Liu, R. Hu, X. Lou, Z. Li, G. Krishnamoorthy, D. Zhang, D. Pasini, Y. Tang, Y. Tsuchiya, Y. Wang, W. Z. Yuan, B. Z. Tang, D. Ding, H. Tian, M.-Q. Zhu, J. Z. Sun, W. Tian, G. Kuang, L. Wu, J. Chen, R. Zhang, T. Jadhav, Faraday Discuss. 2017, 196, 403-414. doi: C7FD90002C |
|
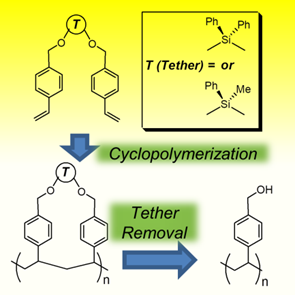
106. ‘The efficient cyclopolymerization of silyl-tethered styrenic difunctional monomers‘ N. Ferri, GB. Ozaydin, A. Zeffiro, A. Nitti, V. Aviyente, D. Pasini,* J. Polym. Sci. Part A, 2018, 56, 1593–1599. DOI: 10.1002/pola.29044. |
|
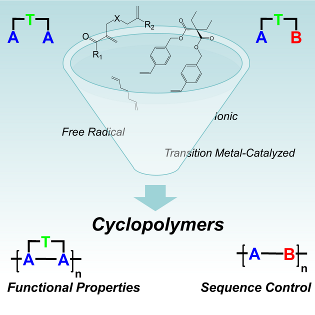
107. ‘Cyclopolymerizations: Synthetic tools for the precision synthesis of macromolecular architectures‘ D. Pasini,* D. Takeuchi,* Chem. Rev., 2018, 118, 18, 8983-9057. DOI: 10.1021/acs.chemrev.8b00286. |
|
109. ‘Visible light 3D printing with epoxidized vegetable oils‘ D. S. Branciforti, S. Lazzaroni, C. Milanese, M. Castiglioni, F. Auricchio, D. Pasini, D. Dondi,* Additive Manuf. 2019, 25, 317–324. DOI:10.1016/j.addma.2018.11.020. |
|
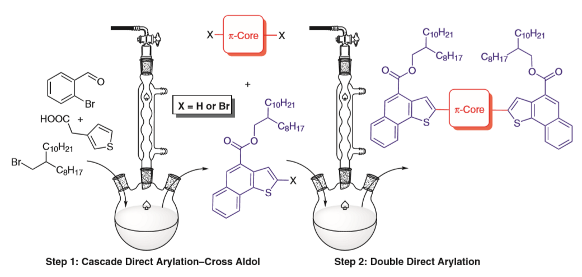
108. ‘Scalable Synthesis of Naphthothiophene-based D-π-D Extended Oligomers through Cascade Direct Arylation Processes‘ A. Nitti, P. Osw, M. N. Abdullah, A Galbiati, D Pasini,* Synlett 2018, 29, 2577-2581. DOI: 10.1055/s-0037-1610331. |
|
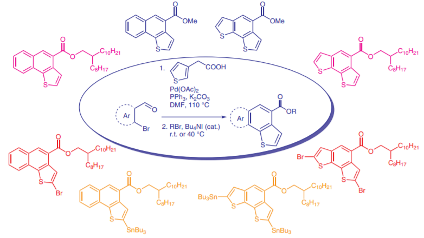
110. ‘Scalable Synthesis of Naphthothiophene and Benzodithiophene Scaffolds as π-Conjugated Synthons for Organic Materials‘ A. Nitti,* G. Bianchi, R. Po, D. Pasini,* Synthesis 2019, 51, 677-682. DOI: 10.1055/s-0037-1611368. |
|
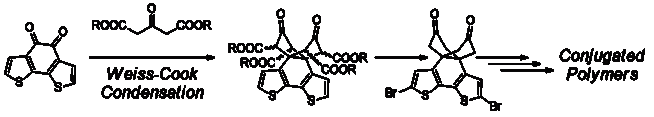
111. ‘Weiss-Cook Condensations for the Synthesis of Bridged Bithiophene Monomers and Polymers‘ A. Nitti, G. Bianchi, R. Po, A. Porta, A. Galbiati, D. Pasini,* ChemistrySelect 2019, 4, 12569–12572. DOI: 10.1002/slct.201904180. |
|
112. ‘Binaphthyl-Based Macrocycles as Optical Sensors for Aromatic Diphenols‘ S. Piacentini, M. Caricato, A. Pacini, A. Nitti, D. Pasini,* Molecules, 2020, 25, 514. Invited Contribution to the Special Issue Molecular Recognition and Self-Assembly in Chemistry and Medicine – Open Access Publication Fees Waived. DOI: 10.3390/molecules25030514. |
|
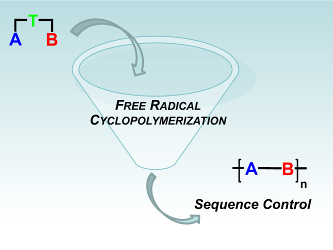
113. ‘Free radical cyclopolymerization: A tool towards sequence control in functional polymers‘, A. Nitti, D. Pasini,* Eur. Polym. J. 2020, 122, 109378. DOI:10.1016/j.eurpolymj.2019.109378. |
|
114. ‘Aggregation-Induced Circularly Polarized Luminescence: Chiral Organic Materials for Emerging Optical Technologies’, A. Nitti, D. Pasini,* Adv. Mater. 2020, 1908021. Invited Contribution to the Special Issue Emerging Chiral Materials. DOI: 10.1002/adma.201908021. |
|
115. ‘Synthesis and Evaluation of Scalable D-A-D π-Extended Oligomers as p-Type Organic Materials for Bulk-Heterojunction Solar Cells’, P. Osw, A. Nitti, M. N. Abdullah, S. I. Etkind, Jeremiah Mwaura, A. Galbiati, D. Pasini,* Polymers 2020, 12, 720. Invited Invited Contribution to the Special Issue Synthesis, Characterization and Simulation of Soft Matter with EUSMI – Open Access Publication Fees Waived. DOI: 10.3390/polym12030720. |
|
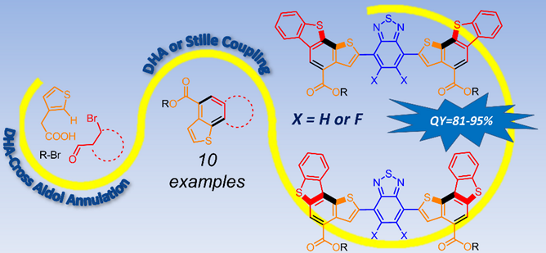
116. ‘One-Pot Regiodirected Annulations for the Rapid Synthesis of π-Extended Oligomers’, A. Nitti, P. Osw, G. Calcagno, C. Botta, S. Etkind, G. Bianchi, R. Po, T. M. Swager, D. Pasini,* Org. Lett. 2020, 22, 3263-3267. DOI: 10.1021/acs.orglett.0c01043. |
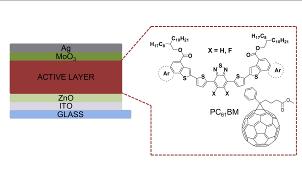
|
117. Chiral Triptycenes in Supramolecular and Materials Chemistry, G. Preda, A. Nitti, D. Pasini,* ChemistryOpen 2020, 9, 719–727. DOI: 10.1002/open.202000077. |